For the Patient
This page is a resource for better understanding the process of being a clinical trial patient and what that entails. Here you’ll find tools that you can use to talk to someone with Angelman Syndrome about clinical trials including videos, story boards and more. Check back frequently for additional content.
Is Clinical Research Right for Me?
Deciding if clinical research is the right step for anybody can seem daunting, that’s why education is key in making an informed decision. These short videos, from hhs.gov, provide basic information about human research, including clinical trials, medical research, and other kinds of research. They help potential research volunteers understand how research works, what questions they should ask, and things to think about when deciding whether to participate in a study.
Part 1
What is Medical Research?
Part 4
Explaining Randomization in Clinical Trials
Part 2
Deciding to Participate in Clinical Trials
Part 5
How is Medical Research Different
from Medical Care?
Part 3
Questions to Ask Before Volunteering
in Clinical Trials
What Questions Should I Ask?
Anyone interested in participating in a clinical study should know as much as possible about the study and feel comfortable asking the research team questions about the study, the related procedures, and any expenses. The following questions may be helpful during such a discussion. Answers to some of these questions are provided in the informed consent document. Many of the questions are specific to clinical trials, but some also apply to observational studies.
Will hospitalization be required?
How long will the study last?
Who will pay for my participation?
Will I be reimbursed for other expenses?
What type of long-term follow-up care is part of this trial?
If I benefit from the intervention, will I be allowed to continue receiving it after the trial ends?
Will results of the study be provided to me?
Who will oversee my medical care while I am participating in the trial?
What are my options if I am injured during the study?
What is an observational study versus an interventional study?
What are possible adverse events?
What is being studied?
Why do researchers believe the intervention being tested might be effective?
Why might it not be effective? Has it been tested before?
What are the possible interventions that I might receive during the trial?
How will it be determined which interventions I receive (for example, by chance)?
Who will know which intervention I receive during the trial? Will I know? Will members of the research team know?
How do the possible risks, side effects, and benefits of this trial compare with those of my current treatment?
What will I have to do?
What tests and procedures are involved?
How often will I have to visit the hospital or clinic?
Source: National Institute of Health
Research Related Procedures
There are a few procedures found in Angelman syndrome clinical trial protocols. One is taking a pill or capsule by mouth. Researchers ask participants to take medicine orally (by mouth) so they can identify the most effective dose for the participant.
Another method is through Intrathecal administration. This is a route of administration for drugs via an injection into the spinal canal, or into the subarachnoid space so that it reaches the cerebrospinal fluid.
Several recent studies want to measure protein levels in cerebrospinal fluid (CSF) with a goal of using those levels as a biomarker for future research. CSF is fluid that surrounds the brain and spinal cord and serves as a cushion to protect the brain from injury. To get a sample of CSF, medical professionals use a couple of methods:
Lumbar puncture/spinal tap
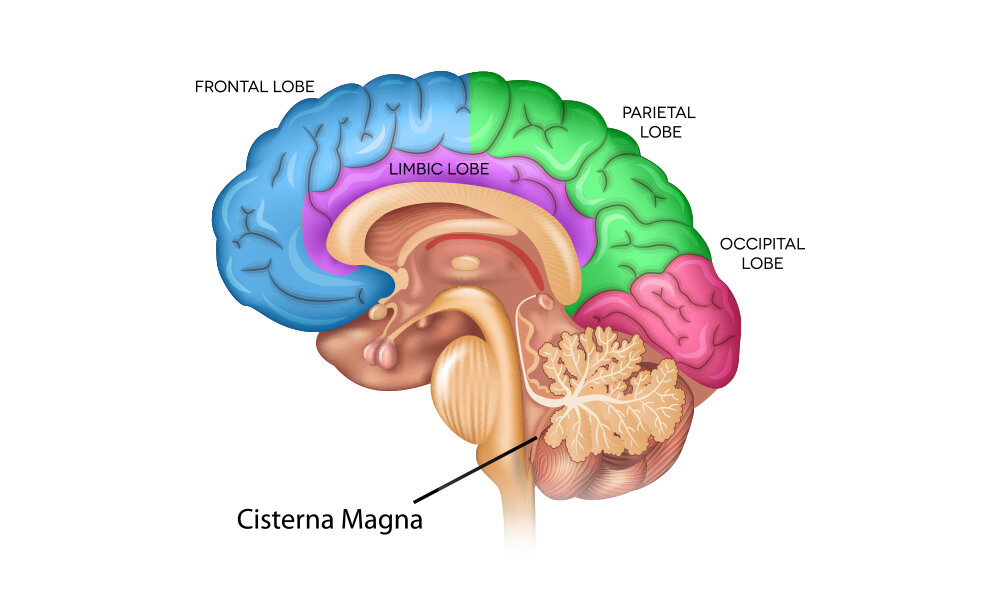
Cisterna magna (CM) injection
Cerebrospinal Fluid (CSF) Procedures
Lumbar Puncture
Lumbar puncture, also called a spinal tap, involves placing a needle between two bones in the spine and removing a sample of CSF. You can learn more about the procedure in the video.
Cisterna Magna Injections
Cisterna magna injections can serve two purposes. The first is to gather a sample of CSF. In this procedure, medical professionals place a needle near the base of the skull and remove fluid for testing. Researchers prefer to use the area near the cisterna magna because it offers a route of entry that does not involve surgery.
The second purpose involves testing the effects of new medication by injecting it into the cisterna magna. Because CSF travels through the entire body, putting a drug directly into the CSF may be the quickest and best delivery system for a new treatment.
These procedures are considered invasive, meaning something is placed inside your body then removed. To make patients more comfortable, the researchers give them medicine before the procedure that makes them sleepy and relaxed. This is called anesthesia.
Lumbar Puncutre
Video Credit: Design Science (please note that most lumbar puncture’s will happen under sedation)
Anesthesia Procedure
Video Credit: Johns Hopkins Medicine
View a 3D Model of the Human Brain.
Brought to you by BrainFacts.org.